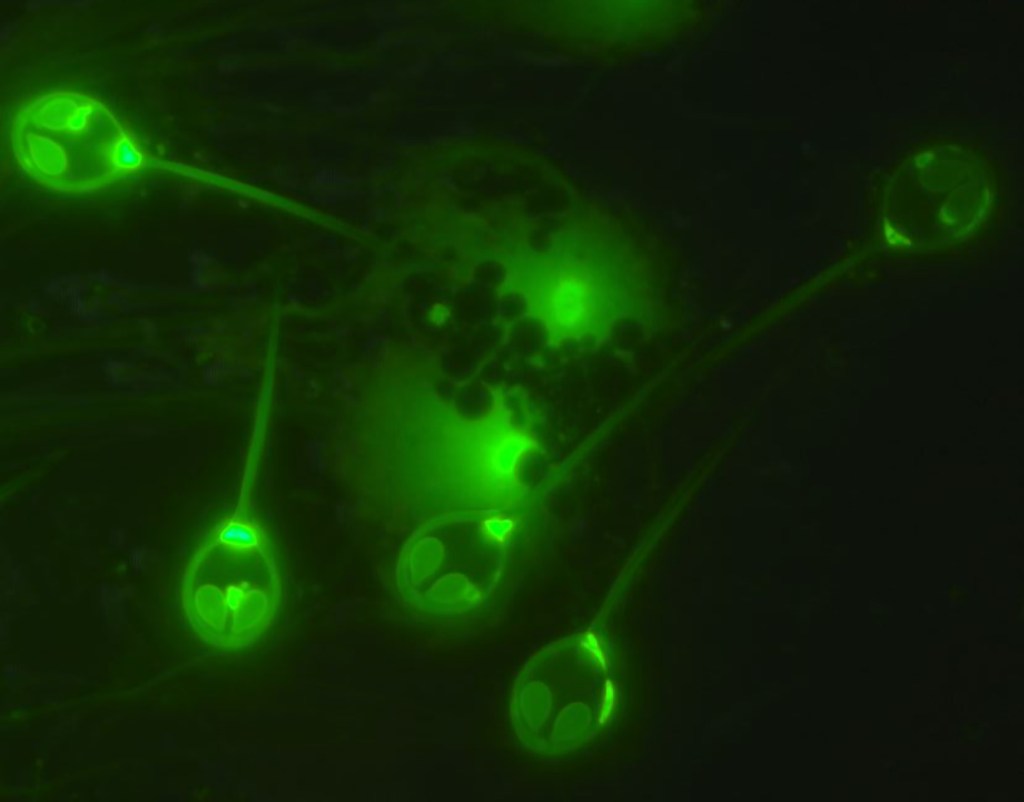
Scientists have discovered the first multicellular creature that not only doesn’t need oxygen to live, but can’t breathe even if it wanted to.
Maybe you remember this from high school biology: mitochondria are the powerhouses of the cell. That’s because of the key roles they play in breaking down nutrients and converting them into energy-rich molecules. This process is called aerobic respiration, and it’s the reason every animal has to breathe air—or so scientists thought.
Videos by VICE
Researchers have discovered an animal lacking a mitochondria and many of the genes that facilitate aerobic respiration. The animal is a salmon parasite called Henneguya salminicola, and it is a member of the phylum Cnidaria, which also contains jellyfish and sea anemones. The international team of researchers published a study with their results on Monday in the journal PNAS.
“Our discovery shows that aerobic respiration, one of the most important metabolic pathways, is not ubiquitous among animals,” the authors wrote in the study.
The history of the mitochondria is inextricably wrapped up in the history of eukaryotic organisms, the domain of organisms that includes all plant and animal life. Due to a set of similarities between mitochondria and bacteria, scientists believe that at least 1.45 billion years ago, a proto-mitochondrion was swimming around doing its thing when it was absorbed into another organism. Instead of digesting it, the organism kept it around and the two cells worked together in a mutually beneficial relationship.
H. salminicola is a micrometer-sized parasite that infects salmon and causes distinctive pseudocysts in the skeletal muscle of salmon. The parasite is commonly found in Alaska, and it causes a condition called “tapioca disease” or “milky flesh disease,” according to a field guide compiled by the Alaska Department of Fish and Game.
“The spores of this parasite occur in the muscle and under the skin of Pacific salmon causing a condition known as ‘milky flesh’ disease because of the creamy white fluid containing spores that oozes from the cysts (pansporoblasts) during filleting,” the field guide reads.
The researchers obtained samples of H. salminicola from Chinook and Coho salmon in Oregon and sequenced their genes along with those of Myxobolus squamalis, a close parasite relative. They looked at parasites from each species under a microscope after adding fluorescent dye that would bind to wherever the mitochondria were. To their surprise, the mitochondria of M. squamalis were clearly visible as small blue dots, but H. salminicola didn’t appear to have any mitochondria.
The genetic sequencing results painted a similar picture. M. squamalis had at least 41 genes likely used for mitochondrial processes, while H. salminicola only had six of these genes. One gene critical for replicating mitochondria had been turned into a pseudogene, making it functionally useless. H. salminicola also lacked many of the proteins that its relatives use in aerobic respiration.
Study senior author Dorothée Huchon said in an email that the discovery of an animal that could not undergo aerobic respiration was pure luck, as she had set out to sequence mitochondria across Myxozoa, the class of parasites to which H. salminicola belongs. She obtained frozen samples of myxozoans from co-author Stephen Atkinson’s lab in Oregon, and one of the samples happened to be H. salminicola.
“My goal was to assemble mitochondrial genome to study its evolution in Myxozoa and… Oops, I found one without a genome,” she said.
At first, Huchon thought it might be a mistake. “I first thought that the lack of mitochondrial genome among the DNA sequence was the result of a bug in genome analyses. But then I realized that it has lost not just the mitochondrial genome but the whole set of protein genes that interact with the mitochondrial genome and all the majority of genes involved in respiration.”
She added that Atkinson then performed the microscopy, which confirmed the molecular results and was “a relief.”
Of course, H. salminicola must have some way of breaking down nutrients. The scientists found mitochondria-related organelles when they viewed H. salminicola under an electron microscope. These organelles contained special folds called cristae that mitochondria also possess to increase the surface area where chemical reactions can take place; however, the ways in which these mitochondria-related organelles function are unknown, since the parasites were dead for these analyses. Additionally, the authors reported that it is “currently not possible” to culture H. salminicola in laboratory settings. Huchon said that this is because the parasite needs two hosts during its life cycle—a fish and a worm—and scientists do not yet know which worm it infects.
In the paper, the authors wrote that future experiments are needed to better understand H. salminicola’s metabolism. Still, the discovery underscores the ingenuity of myxozoans, the class of parasites to which H. salminicola belongs.
“Myxozoans have gone through outstanding morphological and genomic simplifications during their adaptation to parasitism from a free-living cnidarian ancestor,” the authors wrote. “As a highly diverse group with >2,400 species, which inhabit marine, freshwater, and even terrestrial environments, evolutionary loss and simplification has clearly been a successful strategy for Myxozoa, which shows that less is more.”
Like most evolutionary rulebreakers, H. salminicola goes against the definition of a eukaryote in unique and specific ways that make us question the rules in the first place, and appreciate the diversity of life on Earth.
Update: This article was updated with comment from study senior author Dorothée Huchon.
More
From VICE
-

Screenshot: IRONMACE -

Screenshot: Square Enix Collective -

Screenshot: Matt Vatankhah -

Getty Images