Yesterday, Allergan issued a voluntary recall for close to 170,000 sample packs of Taytulla birth control pills because of the possibility that the first four pills in the pack are inactive, which could put people at risk for unintended pregnancies.
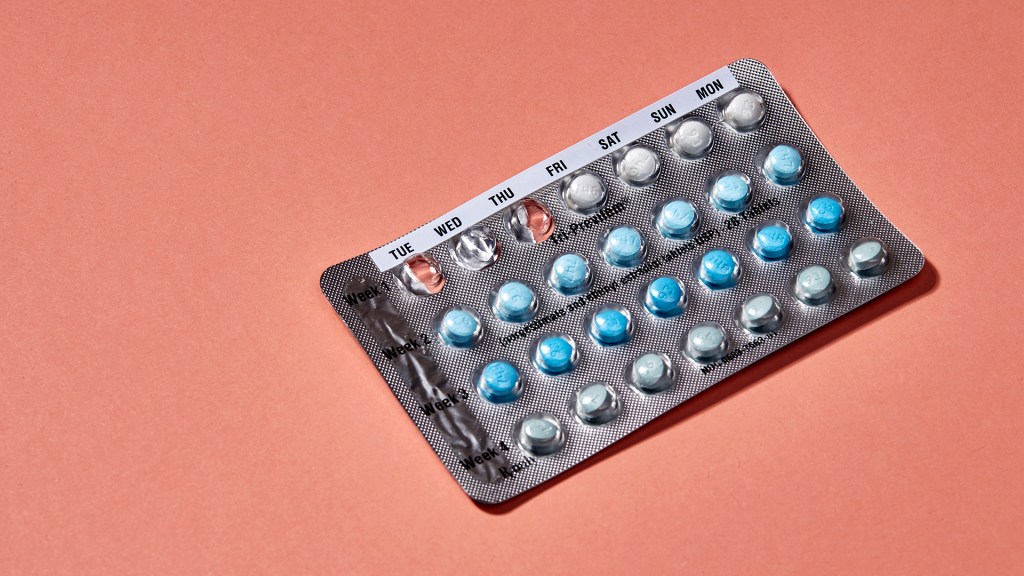
The company said in a statement that a packaging error led to the four maroon placebo pills being inserted at the beginning of a sample pack, rather than at the end, after the 24 pink pills that contain hormones. These hormones prevent the ovaries from releasing an egg, thereby preventing pregnancy. Missing a few days of the active pills, as you would if you took placebo pills, could result in the ovaries releasing an egg.
Videos by VICE
If a couple isn’t using backup birth control like condoms in this instance, they could get pregnant. This is why doctors and pill makers tell people that, after missing two days of active pills, they should use a backup method or abstain from sex until they’ve been back on their pill-popping schedule for seven consecutive days.
The company said that oral contraceptives taken out of sequence “may place the user at risk for contraceptive failure and unintended pregnancy.”
The recall affects 168,768 Taytulla packs from lot #5620706 with an expiration date of May 2019, which were sent as samples to physicians’ offices nationwide starting in August 2017. A doctor reported the packaging error in one sample pack and the company chose to recall the entire lot. The fact that they’re samples means that people may have never taken the pills before and might not know what the packs are supposed to look like.
The recalled pill packs look like this:
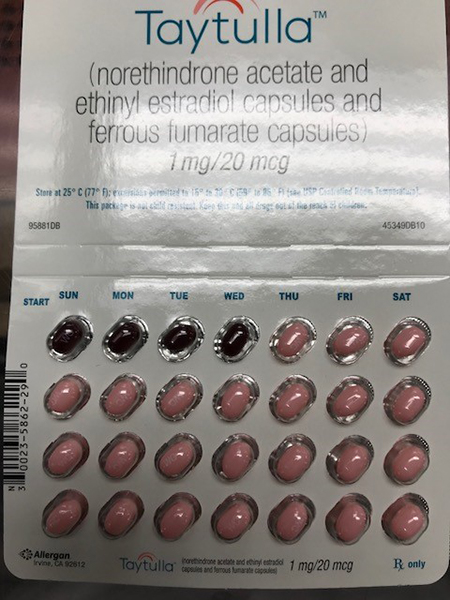
The packs are supposed to look like this:

A company spokesperson tells Tonic that it hasn’t received any reports of unintended pregnancies as a result of the error and that, after an investigation at the packaging site, no other Taytulla sample packs have been identified with the packaging error within the recalled lot, or within any other lot.
Allergan said in a statement that it is arranging for the return of all pill packs from the affected lot and that people who are concerned that they’ve been affected by the packaging error should contact their doctor. The company added that people can report “adverse events” (like pregnancy) to the Food and Drug Administration’s MedWatch program.
Birth control packaging errors aren’t common but they do happen. Last June, Lupin Pharmaceuticals announced a recall of a chewable birth control pill for the same reason: The placebo pills were at the start of the pack, not the end. A similar packaging error resulted in a 2015 lawsuit against Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
Sign up for our newsletter to get the best of Tonic delivered to your inbox.